Key takeaways:
- 81 cases of highly drug-resistant bacterial infections linked to contaminated eyedrops, including four deaths and additional cases of vision loss
- CDC recalled three products in February, including EzriCare Artificial Tears
- CDC urging people to stop using any eyedrops from EzriCare or Delsam Pharma and to contact their healthcare provider if they have any symptoms of an eye infection
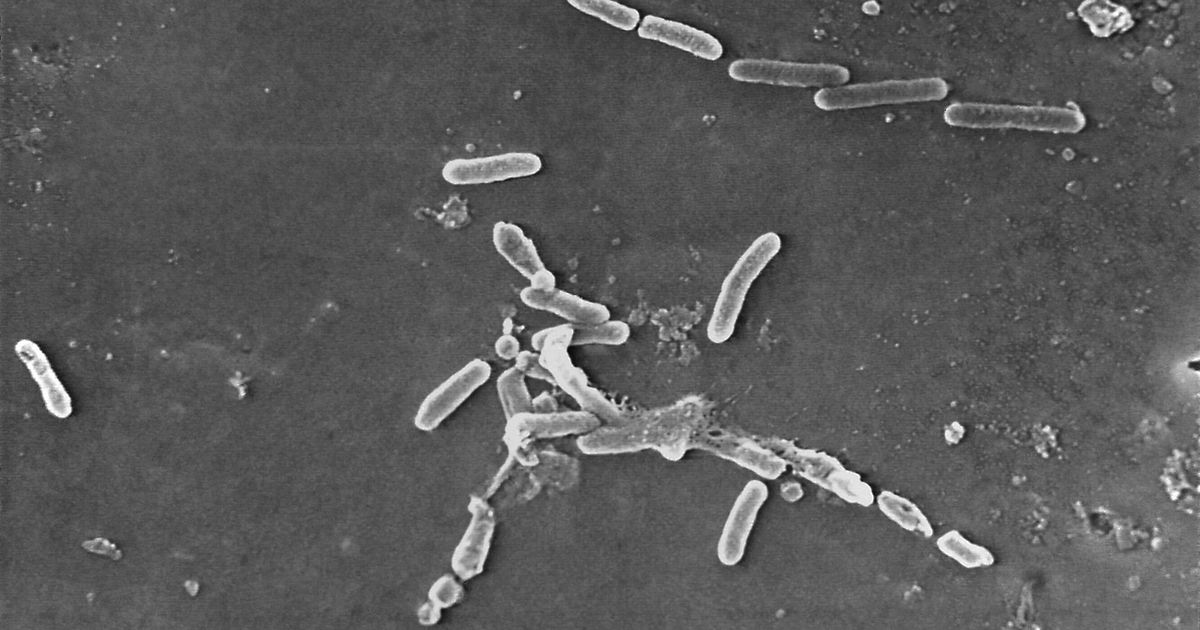
The U.S. Centers for Disease Control and Prevention (CDC) has reported 81 cases of highly drug-resistant bacterial infections linked to contaminated eyedrops, including four deaths and additional cases of vision loss. The CDC recalled three products in February, including EzriCare Artificial Tears, a preservative-free, over-the-counter product that comes in multi-dose bottles.
Maroya Spalding Walters, who leads the CDC’s antimicrobial resistance team, said in an interview that the infections were “catastrophic and life-altering.” Many patients reported using multiple brands of eyedrops, with EzriCare Artificial Tears being a common brand among those affected.
The CDC is urging people to stop using any eyedrops from EzriCare or Delsam Pharma and to contact their healthcare provider if they have any symptoms of an eye infection, such as redness, pain, or discharge. The agency is also recommending that people discard any unused eyedrops from these brands.
The CDC is working with the Food and Drug Administration to investigate the cause of the contamination and to identify any other products that may be affected. In the meantime, the agency is warning people to be aware of the potential risks associated with using these products.


Be First to Comment