Key takeaways:
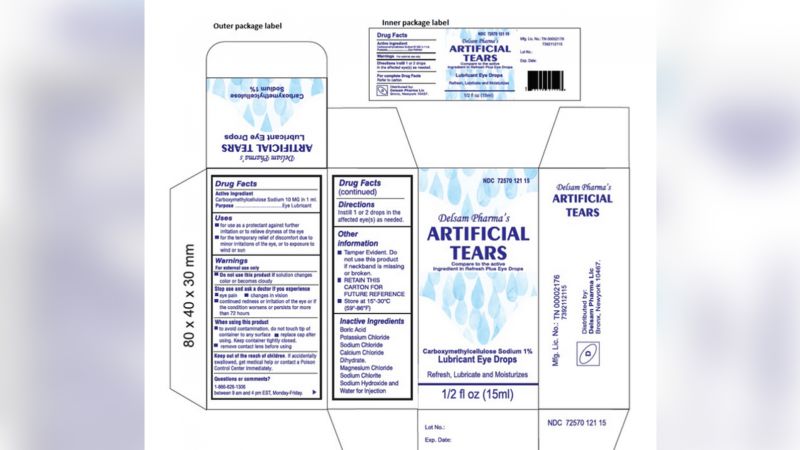
- The US Centers for Disease Control and Prevention (CDC) is warning people to stop using the over-the-counter eye drops, EzriCare Artificial Tears, due to an outbreak of drug-resistant infections of Pseudomonas aeruginosa.
- The CDC has received reports of 55 infections in 12 states, including one fatality.
- The CDC is currently investigating the outbreak and is urging people to stop using the eye drops immediately, and those who have used the product in the last three months should contact their healthcare provider to discuss the risks of infection.
The US Centers for Disease Control and Prevention (CDC) is warning people to stop using the over-the-counter eye drops, EzriCare Artificial Tears, due to an outbreak of drug-resistant infections of Pseudomonas aeruginosa.
The CDC has received reports of 55 infections in 12 states, including one fatality. The infections have been reported in the cornea, intraocular fluids, respiratory tract, urinary tract, and sepsis. Some of the cases have resulted in permanent vision loss or hospitalization.
Global Pharma Healthcare is issuing a recall of its Artificial Tears Lubricant Eye Drops that were distributed by EzriCare and Delsam Pharma due to possible contamination, the US Food and Drug Administration said Thursday.
The CDC is currently investigating the outbreak and is urging people to stop using the eye drops immediately. People who have used the product and are experiencing any symptoms of infection should seek medical attention.
The CDC is also recommending that people who have used the product in the last three months should contact their healthcare provider to discuss the risks of infection and to determine if any additional testing or treatment is needed.
The FDA is continuing to investigate the source of the contamination and is working with Global Pharma Healthcare to ensure that the product is no longer available for sale.

Be First to Comment