Key takeaways:
- The FDA has proposed a major change to the US approach to COVID-19 vaccines, making it more similar to the annual flu vaccine push.
- The FDA expects to assess circulating strains of the virus that causes Covid-19 at least annually and decide in June which strains to select for the fall season.
- If implemented, the change could simplify the process of getting vaccinated against Covid-19 and help ensure that people are protected against the most common strains of the virus.
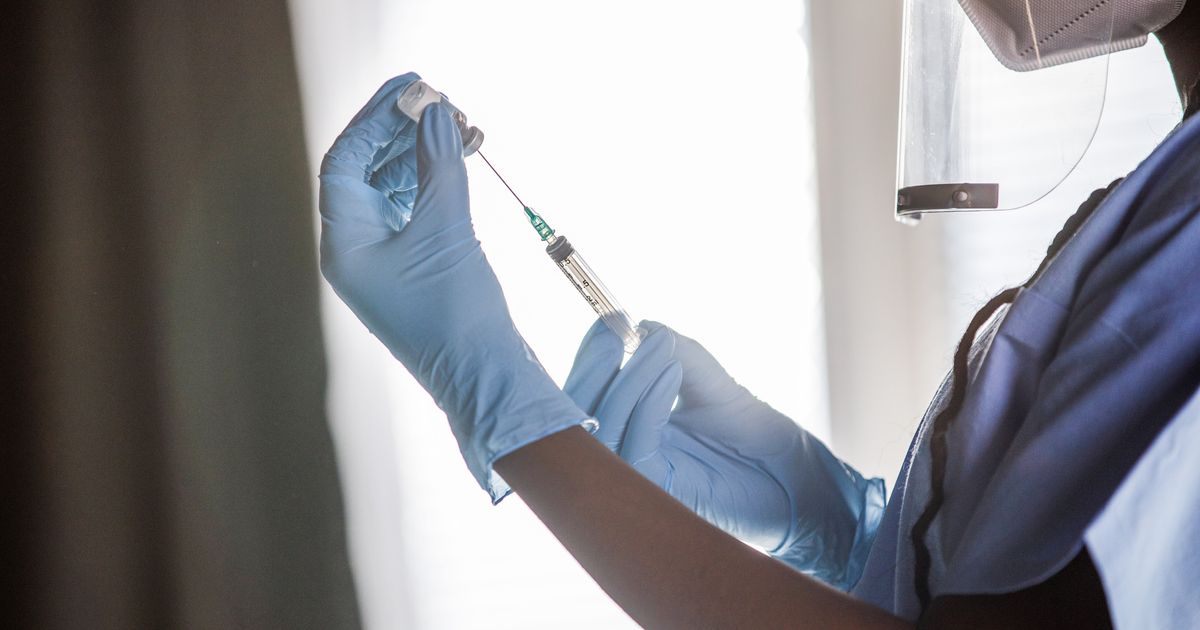
The US Food and Drug Administration (FDA) has proposed a major change to the country’s approach to COVID-19 vaccines that would mirror the strategy behind annual flu shots. The FDA said it expects to assess circulating strains of the virus that causes Covid-19 at least annually and decide in June which strains to select for the fall season. This new plan would empower the FDA to decide in June of each year which strain of the virus is most likely to be the biggest threat the following winter.
The FDA predicts that most people may need only one dose of the latest Covid-19 shot to restore protection, regardless of how many shots they have already received. However, people at high risk of COVID-19 complications may need more than one annual shot.
The FDA’s proposed change to the Covid-19 vaccine process would make it more similar to the annual flu vaccine push. Each year, the flu vaccine is updated to include the most common strains of the virus that are expected to be circulating in the upcoming season. This same approach would be used for the Covid-19 vaccine, with the FDA assessing circulating strains of the virus and deciding which strains to include in the vaccine.
The FDA’s proposed change to the Covid-19 vaccine process is still under review and will be discussed at the agency’s vaccine advisory committee meeting on Thursday. If implemented, the change could simplify the process of getting vaccinated against Covid-19 and help ensure that people are protected against the most common strains of the virus.

Be First to Comment